The Mineralogical Barrier in Space
No such thing as a free lunch in the solar system
The concept of the “mineralogical barrier” introduced by Brian Skinner (Skinner, 1976) is fundamentally important for understanding metal resources in space (i.e., pink colors on this periodic table):
Skinner was concerned with the future sustainability of mining on Earth, and what would happen when we use up all the best–highest grade–ores.
If you go out and sample random portions of the Earth’s crust and measure the concentration (or grade) of a major element like iron or aluminum, you get a distribution that looks something like this:
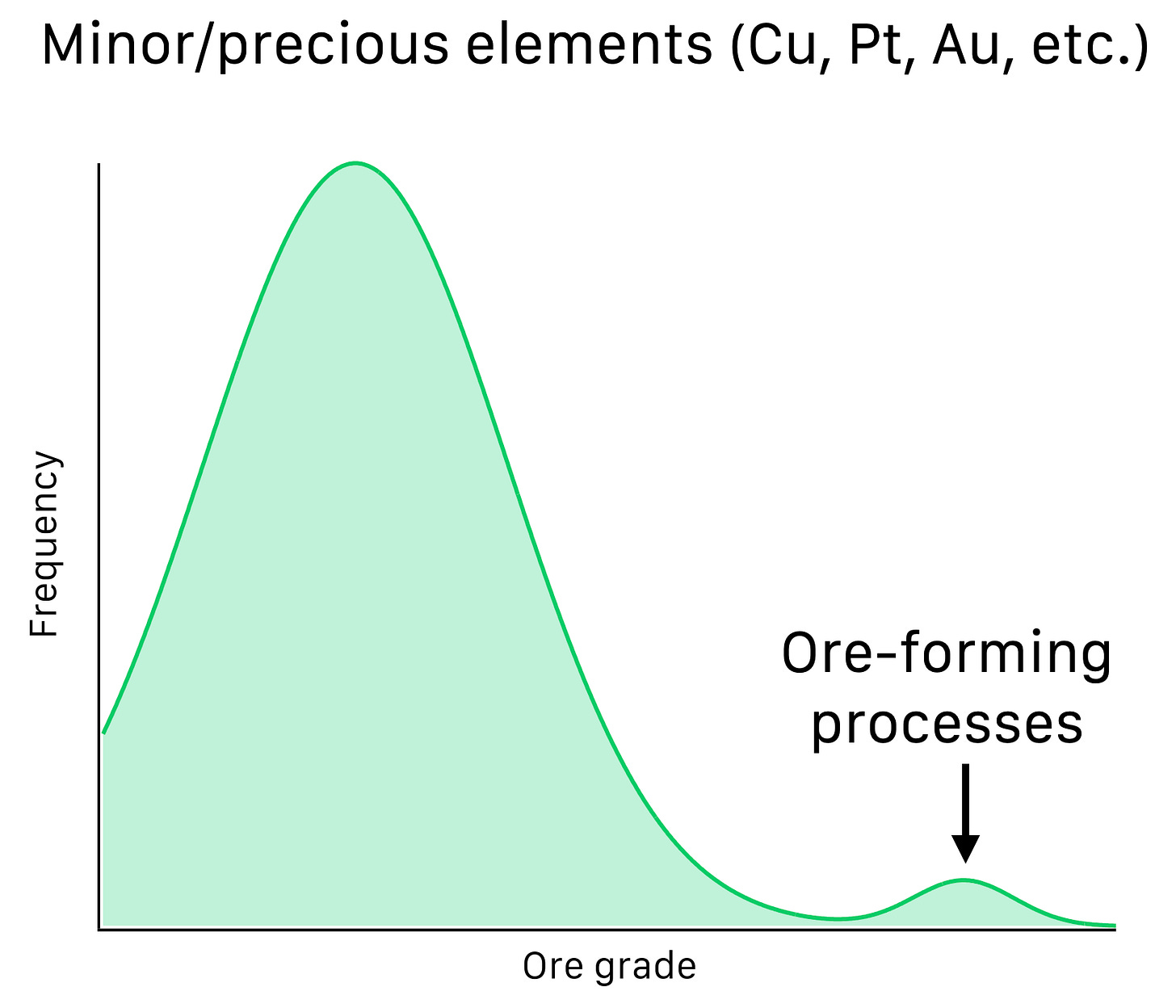
It could be lognormal instead of gaussian, but that’s not important. What Skinner recognized is that for scarce elements like copper or platinum, the distribution is bimodal instead:
The smaller hump on the right with higher grades represents ore bodies that were formed by distinct geologic processes (ore-forming processes) over billions of years.
On Earth, geochemically abundant or major elements are found in specific mineral carriers, where they make up a good fraction of that mineral’s composition. For example, iron is commonly found in the minerals hematite and magnetite among others. Hematite is 70% iron by mass, and magnetite is 72%. In the figure below, this is represented by the element “A” that is a major constituent of Mineral 3.
This is also true for the geochemically scarce elements like copper, but only if we’re sitting in the hump on the right. Otherwise, these elements are dispersed in trace amounts in all sorts of minerals. A single atom here, an atom there. In the figure, this is represented by the element “B”.
Ore-forming processes create valuable minerals (covellite, rutile, etc.) that can be separated from the non-ore minerals (the gangue, or waste) through mineral processing/beneficiation. Doing so greatly increases the concentration of the target element: the fraction of A will go up if we physically separate Mineral 3 from Minerals 1, 2, and 4.
As ore grades go down for abundant metals, the energy and therefore cost to extract metals goes up. Recent work by Valero & Valero has shown this relationship is exponential. But Skinner recognized that if we deplete high-grade ores of scarce metals, there is not a monotonic increase in energy forever, but a distinct gap appears where we can no longer use beneficiation to separate minerals that are enriched in the element we want, because there are no longer such minerals. This is the “mineralogical barrier”:
Recent estimates suggest energy costs may be 2-3 orders of magnitude higher on the wrong side of this barrier. This is the extent of the “free bonus” Earth has given us through plate tectonics and hydrothermal fluids creating ore bodies.
So how does this apply to space resources? Let’s take the Moon for example and consider potential ore-forming processes using the classification from Robb (2020):
We can immediately cross off two entire categories: Hydrothermal, and Sedimentary-surficial which both involve fluid flow. This leaves us with igneous ore-forming processes. But igneous geology is very different on the Moon, and we don’t have super evolved compositions in continental crust that concentrate tin, tungsten, uranium, thorium, etc.
For the majority of elements on the Moon, there simply haven’t been processes to concentrate them and form ores; this has long been recognized with analyses from Apollo data. The same is mostly true for asteroids, and on Mars (although there is greater potential for ore formation on Mars, it likely doesn’t come remotely close to Earth in this respect).
What this means is that a nightmare scenario envisioned for the future of Earth is basically the default we start with in space. We must overcome the mineralogical barrier if we want to expand outwards. One way forward is to simply apply brute force and use the abundant energy in space to get over the barrier. Another is to try to be clever with new technologies.
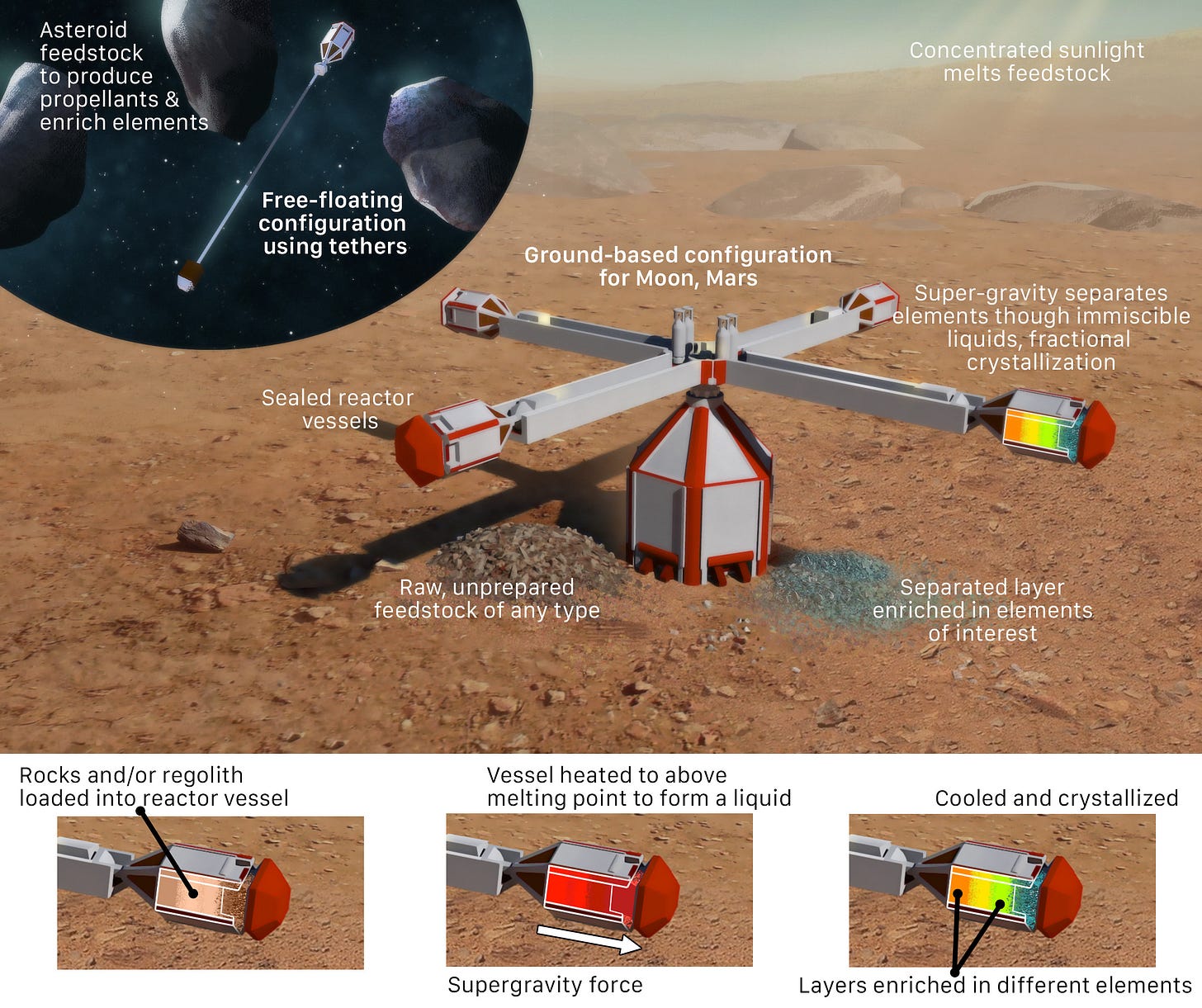
Along these lines, an idea I’ve put forward is to use “synthetic geology” to replicate ore-forming processes, starting with raw regolith and rocks on other planets. The Pinwheel Magma Reactor is a concept for doing this with igneous processes (initially, fractional crystallization), and hydrothermalism can be introduced as a next step:
Obviously, the energy involved in this technology plus the necessary conventional ore extraction steps would have to be less than the brute force energy to rip atoms of say, gold, out of garden variety rocks and regolith.
References
Skinner, B. J. (1976). Second iron age ahead. Am. Sci.;(United States), 64(3).
Robb, L. (2020). Introduction to ore-forming processes. John Wiley & Sons.